Two Foundation programs building diversity in clinical trials - Bristol Myers Squibb - Our stories
BMS Foundation
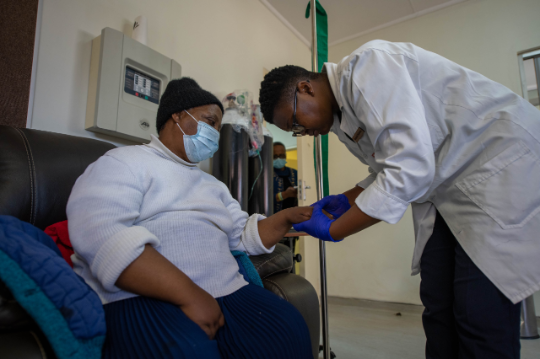
Two Foundation programs building diversity in clinical trials
Conversations about diversity in clinical trials typically focus on ensuring the participants come from a range of social and ethnic backgrounds.
But an often-overlooked step is making sure the medical professionals who conduct the trials also have diverse backgrounds.
The Bristol Myers Squibb Foundation is making headway with its trailblazing Robert A. Winn Diversity in Clinical Trials Award Program, which offers two awards to increase the number of diverse healthcare professionals involved in trials. That, in turn, should help increase the diversity of patients in trials.
 |
It’s groundbreaking work. You don’t often get the opportunity to create a large-scale program with the opportunity for this impact.” | |
Catharine Grimes, Senior Director, BMS Foundation |
Two awards support two types of healthcare providers
The Career Development Award is for early-stage investigator physicians from diverse backgrounds, or those who have a demonstrated commitment to increasing diversity in clinical trials.
The other is the Clinical Investigator Pathway Program, for medical students who are underrepresented in medicine.
The Foundation pledged $100 million to support 250 awards for each program. A $14 million commitment from Gilead Sciences last year will fund 10 additional spots in each category each year for four years. Amgen will contribute $8 million to fund six additional awards from each category over the next three years.
The strength of the program: its unique approach
Winn, the program’s namesake, said the program “unleashed not just a big idea, but a big idea that really is rooted in practicality and the courage of change.”
He said there have been “generations upon generations” of people who have been skilled in the ability to carry out clinical trials. But they haven’t always had the ability to connect with their communities, to understand what existing networks could be leveraged or have the skills or insight on how to be a facilitator and catalyst for bringing people together for clinical trials.
The Winn Award Program changes all that, so far training 114 early-stage investigator physicians and providing 44 medical students with an immersive experience in community-based clinical research.
The program is now set to reach more than 300 community-oriented clinical trialists and 300 underrepresented medical students by 2027.
What’s different?
“We are really training them in a bold new way,” said Catharine Grimes, senior director at the Foundation.
Other programs teach medical professionals how to conduct clinical trials, but Grimes said those programs don’t include education on community outreach and engagement skills. “It doesn’t exist in any medical education that we are aware of,” she said.
Foundation President John Damonti called the effort a “marquee program” for the training community. He said Winn is a “tireless advocate” for the program’s goal of enabling diverse and underserved populations to get more benefit from clinical research.
Award recipients work and learn with top experts
The physicians in the Winn Award Program will not only learn how to develop a clinical trial, but they will also work with some of the country’s leading experts on community outreach engagement and community integration.
“It’s groundbreaking work,” Grimes said. “You don’t often get the opportunity to create a large-scale program with the opportunity for this impact.”
