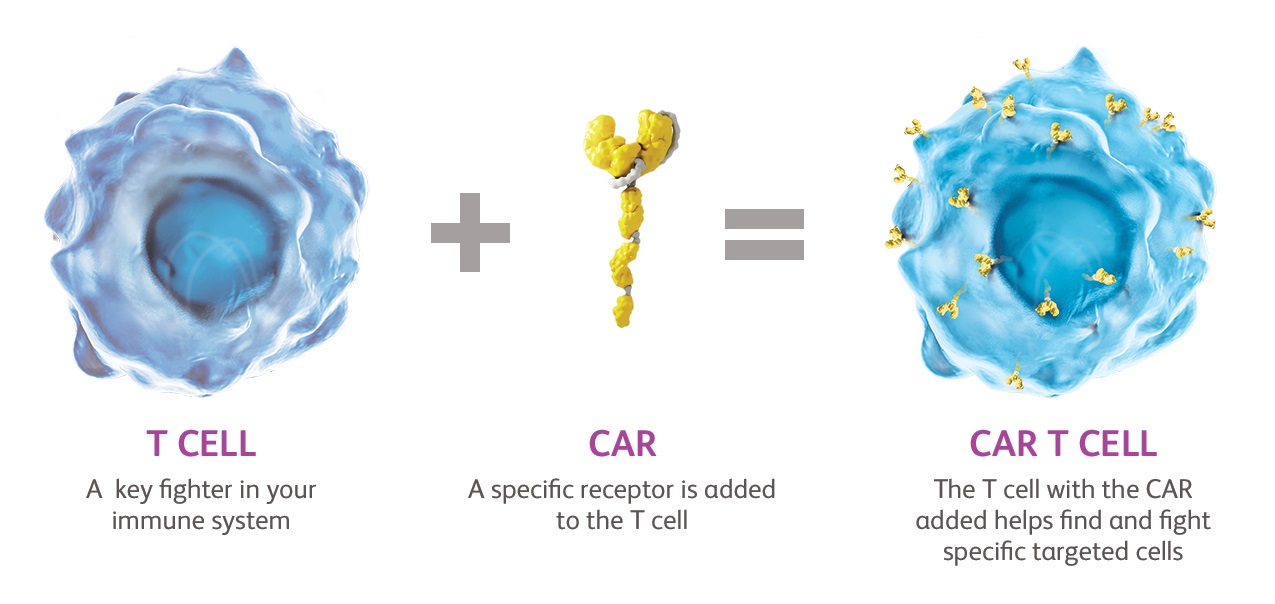
As part of its efforts to deliver cell therapy to patients with haematological cancers in Belgium and the rest of Europe, Bristol Myers Squibb is setting up a brand-new CAR T cell therapy production facility in Leiden, Netherlands. This facility will be equipped with the latest technology and production equipment to develop cell therapy for patients.
Work is currently underway on the design and planning stage of the site in Leiden Bio Science Park. Construction is due to start later this year, and we expect the facility to be completed and in use by the end of 2024. This site will be our fifth production facility across the world developing cell therapy, and our first in Europe.