Understanding targeted protein degradation
Proteins are fundamental to cellular function
Proteins are large, complex molecules that have a range of significant roles in the body and are necessary for the structure, function and regulation of tissues and organs.1
Cells maintain the proper balance of proteins by regulating several fundamental processes including protein synthesis and degradation, or the creation and removal of proteins.2

Protein homeostasis is critical for cell health
Protein degradation is part of a cell’s protein homeostasis regulatory network that ensures unnecessary proteins are removed from the cellular environment when they are no longer needed, are damaged or faulty in some way.2,3
A balanced and efficiently functioning proteome, or all the possible proteins in an organism, is fundamental to all cellular processes and critical to the health of the cell and lifespan of the organism. The accumulation of proteins within a cell is implicated in the pathogenesis of many diseases, including several malignancies and neurodegenerative disorders.2,3

Protein degradation in practice
The ubiquitin-proteasome system (UPS) is one of 2 primary means of protein degradation in cells (the other is lysosomal proteolysis). The ubiquitin ligase enzyme complex, a component of the UPS, tags proteins for degradation with ubiquitin. Ubiquitin-tagged proteins are then sent to the proteasome and degraded.3
Leveraging the protein degradation system to eliminate target proteins
Many current therapeutic approaches focus on inhibiting specific pathways or proteins.
Only a small fraction of target proteins are considered "druggable", or have the potential to bind traditional small molecule therapeutics with high potency.4,5
Scientists are leveraging the body's natural protein degradation system to target and remove proteins influencing disease processes for therapeutic purposes.

By redirecting the UPS within a cell through the introduction of protein degradation agents, scientists may be able to target thousands of previously undruggable proteins or proteins that are chemically intractable by direct pharmacology.6-13
Three approaches to targeted protein degradation
Our scientists are leveraging 3 different modalities of targeted protein degradation – molecular glues, also called CELMoDTM agents, ligand-directed degraders (LDDs) and degrader antibody conjugates (DACs).
All approaches bring the ligase and the intended target protein into close proximity with each other to initiate protein degradation by the cell’s UPS.
This three-pronged approach provides more opportunities for breakthroughs across a broad range of diseases including blood cancers and disorders, solid tumors, immune-mediated diseases and neurological disorders.
Based on our molecular understanding, these 3 approaches work in different ways to redirect the UPS and initiate degradation of the target protein
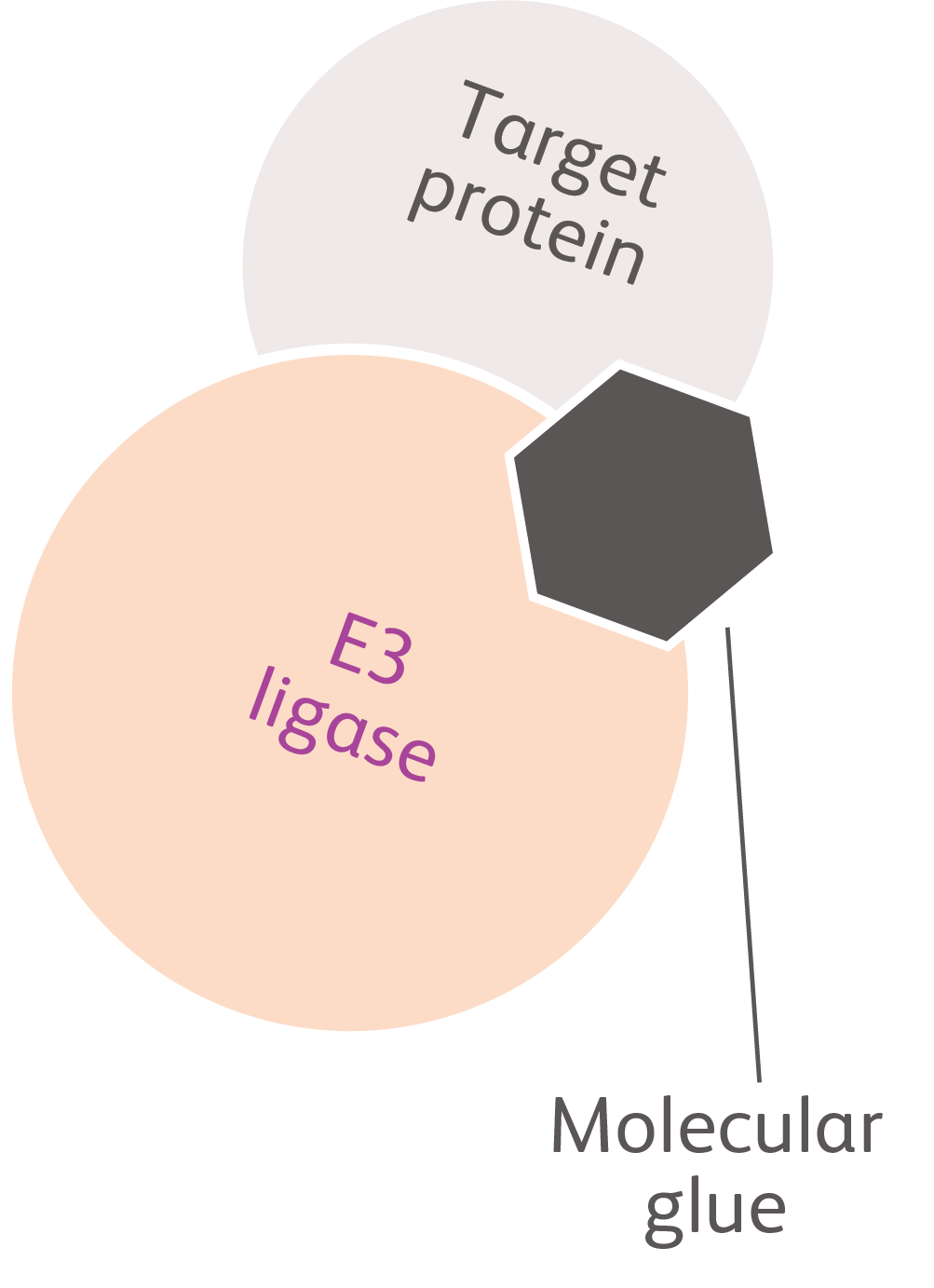
Molecular Glues (CELMoD Agents)Molecules that alter the protein-binding properties of cereblon (an important component of the protein degradation cellular machinery) to promote interaction with and degradation of target proteinsWork by binding to a pocket on cereblon and altering its surface, changing what cereblon is able to “stick to”14,15 |
Watch how degradation with a molecular glue works here |
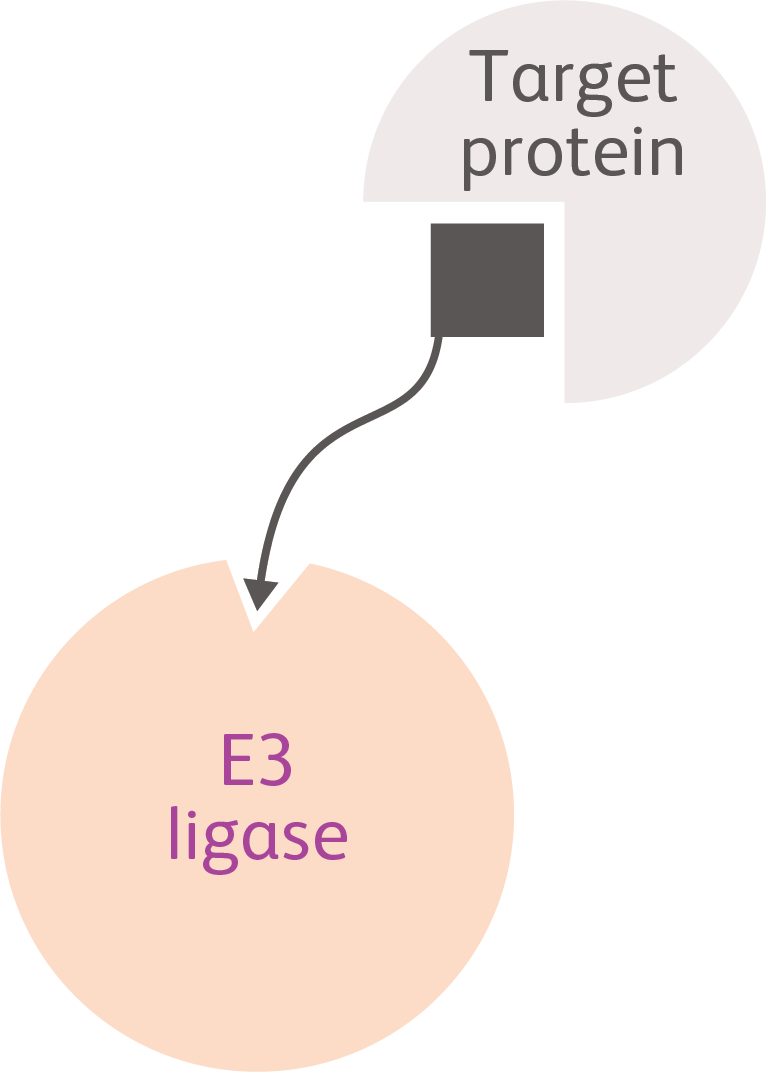
Ligand-directed Degraders (LDDs)
Three-part molecules (2 different ends joined by a linker) engineered to link target proteins with key components of the cellular protein degradation machinery, redirecting the machinery to degrade the target proteins16
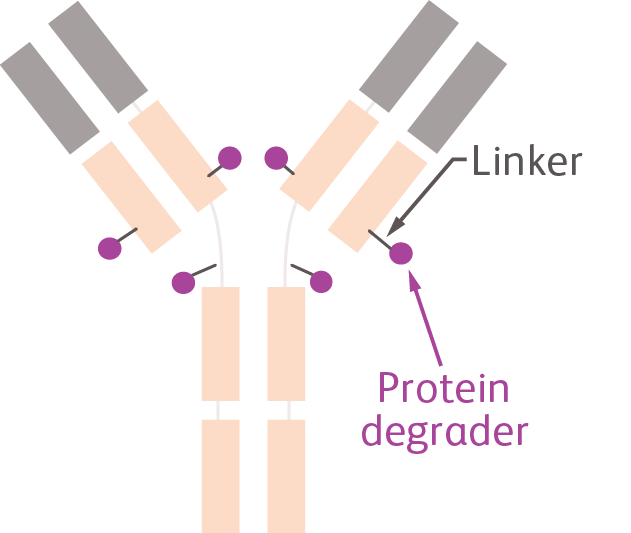
Degrader Antibody Conjugates (DACs)
Consist of 3 elements: a monoclonal antibody that specifically targets cancer cells, the payload (which is a protein degrader) and a linker that connects the two
Bristol Myers Squibb is building on decades of unique research and clinical experience to advance the field of targeted protein degradation and transform patient outcomes in diseases with serious unmet need.
REFERENCES:
- What are proteins and what do they do? - Genetics Home Reference - NIH. U.S. National Library of Medicine. https://ghr.nlm.nih.gov/primer/howgeneswork/protein. Accessed May 7, 2020.
- Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2017;217(1):51-63.
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79-87.
- Oprea TI, Bologa CG, Brunak S, et al. Unexplored therapeutic opportunities in the human genome. Nat Rev Drug Discov. 2018;17(5):317-332.
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727-730.
- Collins I, Wang H, Caldwell JJ, Chopra R. Chemical approaches to targeted protein degradation through modulation of the ubiquitin–proteasome pathway. Biochem J. 2017;474(7):1127-1147.
- Ito T, Ando H, Suzuki T, et al. Identification of a Primary Target of Thalidomide Teratogenicity. Science. 2010;327(5971):1345-1350.
- Krönke J, Udeshi ND, Narla A, et al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science. 2013;343(6168):301-305.
- Lu G, Middleton RE, Sun H, et al. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science. 2013;343(6168):305-309.
- Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4CRBN. Br J Haematol. 2013;164(6):811-821.
- Chamberlain PP, Lopez-Girona A, Miller K, et al. Structure of the human Cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014;21(9):803-809.
- Ito T, Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int J Hematol. 2016;104(3):293-299.
- Matyskiela ME, Lu G, Ito T, et al. A novel cereblon modulator recruits GSPT1 to the CRL4CRBN ubiquitin ligase. Nature. 2016;535(7611):252-257.
- Chamberlain PP, Cathers BE. Cereblon modulators: low molecular weight inducers of protein degradation. Drug Discov Today Technol. 2019;31:29-34. doi:10.1016/j.ddtec.2019.02.004
- Baek K, Schulman BA. Molecular glue concept solidifies. Nat Chem Biol. 2020;16(1):2-3. doi:10.1038/s41589-019-0414-3
- Scheepstra M, Hekking KFW, van Hijfte L, Folmer RHA. Bivalent ligands for protein degradation in drug discovery. Comput Struct Biotechnol J. 2019;17:160-176. doi:10.1016/j.csbj.2019.01.006