A heritage of improving lives through innovation


Explore our history


1819
A LIFETIME OF INNOVATION IN SCIENCE AND MEDICINE BEGINS
Edward Robinson Squibb was born in Wilmington, Delaware, on July 4, 1819.
Early in his life, Edward dreamed of becoming a physician. To make this possible, he served five years as an apothecary’s apprentice in Philadelphia, Pennsylvania, and from his meager pay he saves enough to enter Jefferson Medical College.
1845
EDWARD ROBINSON SQUIBB
Dr. Squibb graduates from Philadelphia’s Jefferson Medical College. He spends a decade as a surgeon in the U.S. Navy, an experience that impresses upon him the urgent need for high-quality, standardized medicines.
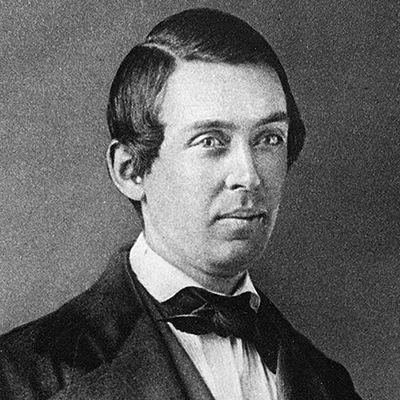
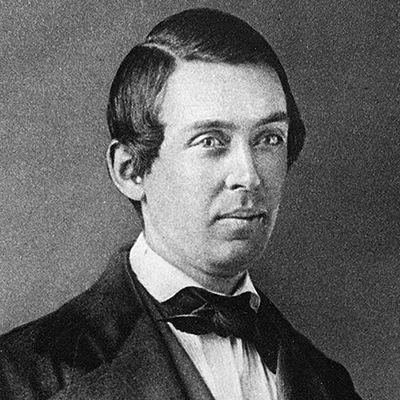
1845
EDWARD ROBINSON SQUIBB
Dr. Squibb graduates from Philadelphia’s Jefferson Medical College. He spends a decade as a surgeon in the U.S. Navy, an experience that impresses upon him the urgent need for high-quality, standardized medicines.


1847
SQUIBB’S QUEST FOR PURITY
Dr. Squibb makes his mark as a forceful crusader against the impure drugs of the times. At the government’s request, he establishes a laboratory for the Naval Hospital in Brooklyn, New York, and serves as its director.
1852
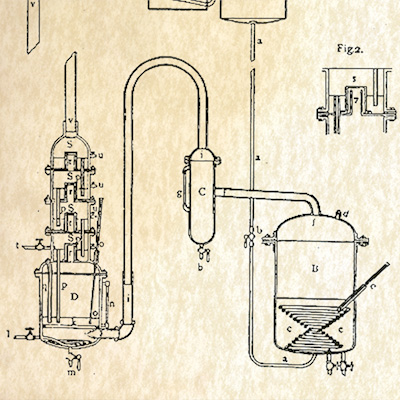
ETHER PRODUCTION
While at the Naval Hospital, Dr. Squibb develops a better way to produce ether and shares his discovery with the rest of the medical community. His success at producing ether using steam distillation helps thousands of surgical patients who were suffering through operations without the benefit of anesthesia. Dr. Squibb is encouraged by the government to establish his own firm to aid the armed forces.

1852
ETHER PRODUCTION
While at the Naval Hospital, Dr. Squibb develops a better way to produce ether and shares his discovery with the rest of the medical community. His success at producing ether using steam distillation helps thousands of surgical patients who were suffering through operations without the benefit of anesthesia. Dr. Squibb is encouraged by the government to establish his own firm to aid the armed forces.


1858
FIRST LABORATORY
Dr. Squibb rents a brownstone in Brooklyn, New York, for his new business and begins pharmaceutical manufacturing operations. In December, he is severely burned in an ether fire that nearly destroys his business.
1860
WILLIAM MCLAREN BRISTOL
Born in Clinton, New York, on July 28, 1860.


1860
WILLIAM MCLAREN BRISTOL
Born in Clinton, New York, on July 28, 1860.


1860
FIRST QUALITY CONTROL LAWS
Dr. Squibb receives appointment to the Committee of Revision of the U.S. Pharmacopeia. He indicates to committee members what formulas can be accepted, improved, or dropped altogether—creating a “quality control” for the first time.
1861–1865
SQUIBB MEDICINE CHEST
During the Civil War, Squibb manufactures compact, portable chests for military surgeons that contain an array of medicines and equipment—from ether and morphine to bandages and sponges. Each chest costs about $100, the equivalent of several thousand dollars today.


1861–1865
SQUIBB MEDICINE CHEST
During the Civil War, Squibb manufactures compact, portable chests for military surgeons that contain an array of medicines and equipment—from ether and morphine to bandages and sponges. Each chest costs about $100, the equivalent of several thousand dollars today.


1862
FIRST DRUG REGULATIONS
With Dr. Squibb’s untiring concern for the use of quality drugs, he justifies his criticism of the American Pharmaceutical Society for its continued failure to enforce the Act of 1848—an Act which prohibits the import of adulterated drugs.
Dr. Squibb expands his business and construction begins on a new laboratory building on nearby Doughty Street in Brooklyn.
1864
JOHN RIPLEY MYERS
Born in Cleveland, Ohio, on October 8, 1864.


1864
JOHN RIPLEY MYERS
Born in Cleveland, Ohio, on October 8, 1864.


1872
SQUIBB’S HIGH STANDARDS
Dr. Squibb’s meticulousness impacts the medicine and pharmaceutical industries at large. His maxim was: “Look at the bottle, look at the label, look before you fill, look when you have filled.”
1879
DR. SQUIBB’S PROPOSAL FOR SAFE MEDICINE
Dr. Squibb presents a report to the New York State Medical Society, “Proposed Law to Prevent the Adulteration of Food and Medicine and to Create a State Board of Health.” Known as the Squibb Bill, it becomes law and serves as the basis for the Federal Pure Food and Drug Act of 1906.


1879
DR. SQUIBB’S PROPOSAL FOR SAFE MEDICINE
Dr. Squibb presents a report to the New York State Medical Society, “Proposed Law to Prevent the Adulteration of Food and Medicine and to Create a State Board of Health.” Known as the Squibb Bill, it becomes law and serves as the basis for the Federal Pure Food and Drug Act of 1906.


1882
SQUIBB MEDICAL JOURNAL
Dr. Squibb introduces a bi-monthly publication called Ephemeris. Written for physicians, the magazine offers information on new pharmaceutical developments. It also attacks what he considers to be “quack” medicine—one of his life-long obsessions.
1887
CLINTON PHARMACEUTICALS
William Bristol and John Myers invest $5,000 to purchase the failing Clinton Pharmaceutical Company. With a pledge to sell no “quack remedies,” the two quickly turn the business around.


1887
CLINTON PHARMACEUTICALS
William Bristol and John Myers invest $5,000 to purchase the failing Clinton Pharmaceutical Company. With a pledge to sell no “quack remedies,” the two quickly turn the business around.


1888
CLINTON PHARMACEUTICAL COMPANY CATALOG
Clinton Pharmaceutical Company promotes 1,800 products in their first catalog, including “gelatin coated pills and granules, compressed tablets, tablet triturates, hypodermic tablets, tinctures, elixirs, wines and syrups.”
1892
E.R. SQUIBB & SONS FORMED
Dr. Squibb forms a partnership with his sons, Edward and Charles, and the firm is renamed E.R Squibb & Sons. The company has capital assets of $1.5 million, according to the Articles of Incorporation filed in 1895.


1892
E.R. SQUIBB & SONS FORMED
Dr. Squibb forms a partnership with his sons, Edward and Charles, and the firm is renamed E.R Squibb & Sons. The company has capital assets of $1.5 million, according to the Articles of Incorporation filed in 1895.

1895
FIRST BREAKTHROUGH PRODUCT
Marketed as a tonic and laxative, Sal Hepatica salts, dubbed the “poor man’s spa,” mimic the taste and effect of the natural mineral waters of a Bohemian spa. Within a decade, annual sales rise to a half-million dollars. The product is a mainstay of Bristol Myers until 1974.


1899
BRISTOL, MYERS COMPANY
Bristol and Myers rename their business the Bristol, Myers Company, which outgrows its home in Clinton, New York. The company relocates — first to Syracuse and then, in 1899, to Brooklyn to better serve customers in New England and Pennsylvania.

1901
IPANA
Bristol-Myers introduces Ipana toothpaste, featuring a disinfectant to prevent tooth decay and gum disease.
1902
SQUIBB QUALITY-CONTROL LABORATORY
E.R. Squibb & Sons establishes its first quality-control laboratory. Soon, it has nearly 100 employees and produces over 800 preparations.


1902
SQUIBB QUALITY-CONTROL LABORATORY
E.R. Squibb & Sons establishes its first quality-control laboratory. Soon, it has nearly 100 employees and produces over 800 preparations.

1905
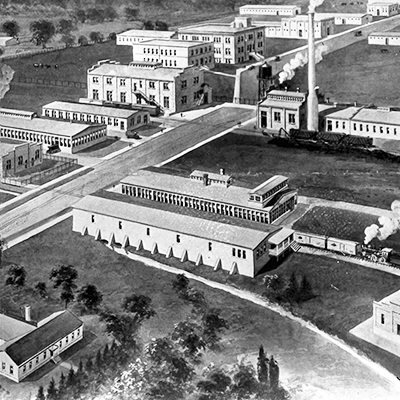
NEW JERSEY PLANT
E.R. Squibb & Sons purchases an ether manufacturing plant in what was then the wilds of New Brunswick, New Jersey.
1906
PURE FOOD AND DRUG ACT
President Theodore Roosevelt signs the Pure Food and Drug Act of 1906 into law. Although Dr. Squibb died in 1900, the act is a triumph of his lifelong crusade for safe, reliable pharmaceutical products.
GROWING IN NEW JERSEY
Bristol-Myers purchases seven acres in Hillside, New Jersey, for expanded production of Sal Hepatica.


1906
PURE FOOD AND DRUG ACT
President Theodore Roosevelt signs the Pure Food and Drug Act of 1906 into law. Although Dr. Squibb died in 1900, the act is a triumph of his lifelong crusade for safe, reliable pharmaceutical products.
GROWING IN NEW JERSEY
Bristol-Myers purchases seven acres in Hillside, New Jersey, for expanded production of Sal Hepatica.


1914
SQUIBB BIOLOGICS
Squibb Biological Laboratories division produces antitoxins, serums and vaccines.
E.R. Squibb & Sons serves as the primary supplier of ether during WWI.

1917
DIGITALIS
E.R. Squibb & Sons pioneers the standardization of digitalis, derived from the foxglove plant as an early treatment for heart conditions.
1919
SQUIBB EXPANSION
E.R. Squibb & Sons product line grows to 2,382 items sold in 6,547 packages, including newly-introduced veterinary medicines. The company expands westward by opening a distribution center in Chicago, and also organizes an export department in Brooklyn—establishing itself as a leader in international trade.


1919
SQUIBB EXPANSION
E.R. Squibb & Sons product line grows to 2,382 items sold in 6,547 packages, including newly-introduced veterinary medicines. The company expands westward by opening a distribution center in Chicago, and also organizes an export department in Brooklyn—establishing itself as a leader in international trade.


1920
SQUIBB COMMITMENT TO BETTER HEALTH
E.R. Squibb & Sons produces vaccines during the first decades of the 20th century, helping to protect an entire generation—while laying the foundation for the modern pharmaceutical industry. These revolutionary vaccines were possible due to the wide acceptance of germ theory in the late 1800s. For the first time, common ailments like diphtheria, tuberculosis, tetanus and typhoid fever could be prevented.
1921
SQUIBB’S “PRICELESS INGREDIENT”
By 1921, E.R. Squibb & Sons produces over-the-counter home remedies like bicarbonate of soda, castor oil, milk of magnesia and Epsom salts.
An advertising campaign is now needed to reach consumers, without offending the publicity-shy medical profession.
A young copywriter hits on the concept “Priceless Ingredient,” and unveils a classic of American advertising.


1921
SQUIBB’S “PRICELESS INGREDIENT”
By 1921, E.R. Squibb & Sons produces over-the-counter home remedies like bicarbonate of soda, castor oil, milk of magnesia and Epsom salts.
An advertising campaign is now needed to reach consumers, without offending the publicity-shy medical profession.
A young copywriter hits on the concept “Priceless Ingredient,” and unveils a classic of American advertising.


1929
BRISTOL-MYERS GOES PUBLIC
Gross profits top $1 million and the company’s products are sold in 26 countries. Bristol Myers becomes a publicly held firm on the New York Stock Exchange.
1938
SQUIBB INSTITUTE FOR MEDICAL RESEARCH
Squibb furthered its commitment to innovative research by opening the 52,000-square foot Squibb Institute for Medical Research in New Brunswick, New Jersey.


1938
SQUIBB INSTITUTE FOR MEDICAL RESEARCH
Squibb furthered its commitment to innovative research by opening the 52,000-square foot Squibb Institute for Medical Research in New Brunswick, New Jersey.

1939
INTOCOSTRIN
Squibb researchers develop a new method for preparing curare and provide free samples of Intocostrin to clinical researchers, who find the curare extract helpful in relaxing the muscles of surgical patients.


1943
CHEPLIN BIOLOGICAL LABORATORIES
Bristol-Myers acquires Cheplin Laboratories in Syracuse, New York, enabling it to mass produce penicillin during World War II. This marks the company’s entry into ethical drug development.
1944
PENICILLIN PRODUCTION PLANT
Squibb opens the world’s largest penicillin production facility in New Brunswick, New Jersey.
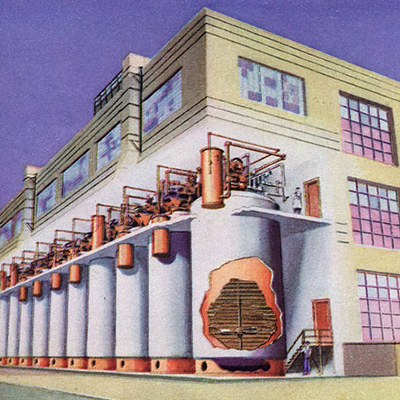

1944
PENICILLIN PRODUCTION PLANT
Squibb opens the world’s largest penicillin production facility in New Brunswick, New Jersey.
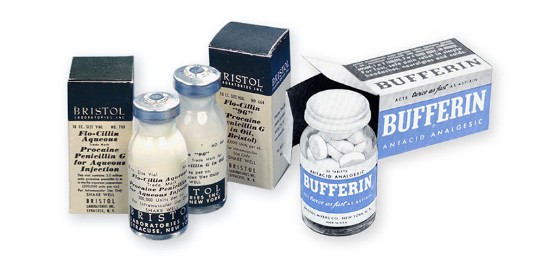
1948
FLO-CILLIN ‘96’
Bristol Laboratories introduces Flo-Cillin 96, a long-lasting injectable penicillin.
NEW PAIN RELIEVER
Bufferin is also introduced.


1955
SQUIBB RECEIVES THE LASKER AWARD
Squibb and Hoffman-La Roche share the Lasker Award for their work on the anti-tubercular agent isoniazid. This is the first time the Lasker Award is bestowed on the pharmaceutical industry.
1956
EARLY CANCER RESEARCH
Bristol-Myers enters the cancer drug development field, building on collaborations around promising antibiotics with the Microbial Chemistry Research Foundation of Japan.


1956
EARLY CANCER RESEARCH
Bristol-Myers enters the cancer drug development field, building on collaborations around promising antibiotics with the Microbial Chemistry Research Foundation of Japan.


1959
CLAIROL PURCHASED
Bristol-Myers acquires the hair-coloring company Clairol, which was founded in 1931 by Joan and Lawrence M. Gelb. Their son, Richard L. Gelb, would later serve as Bristol Myers’ president, CEO and Chairman of the Board.
1966
NEW LABORATORY
Ground is broken for the construction of Bristol-Myers Products research and development laboratories in Hillside, New Jersey. It is the largest proprietary laboratory in the world.


1966
NEW LABORATORY
Ground is broken for the construction of Bristol-Myers Products research and development laboratories in Hillside, New Jersey. It is the largest proprietary laboratory in the world.


1967
MEAD JOHNSON & COMPANY
Bristol-Myers acquires Mead Johnson, maker of Enfamil baby formulas and other nutritional products. Pharmaceuticals included early anti-cancer drugs Megace and Cytoxan.
1967
Anti-Cancer Treatment
Squibb researchers develop HYDREA® (hydroxyurea), the company’s first anti-cancer treatment.
1971
SQUIBB HEADQUARTERS ESTABLISHED
Squibb establishes worldwide headquarters in Princeton, New Jersey, and expands facilities for the Squibb Institute for Medical Research. This allows Squibb to make more groundbreaking discoveries and advancements.


1971
SQUIBB HEADQUARTERS ESTABLISHED
Squibb establishes worldwide headquarters in Princeton, New Jersey, and expands facilities for the Squibb Institute for Medical Research. This allows Squibb to make more groundbreaking discoveries and advancements.


1972
PALOMAR PICTURES INTERNATIONAL
Bristol-Myers announces the formation of Palomar Pictures International. Palomar produces several films, including The Stepford Wives and The Taking of Pelham One Two Three. The company exits the film business two years later.
1973
PRODUCT APPROVAL
Bristol Laboratories introduces MUTAMYCIN® (mytomycin) for the treatment of bone cancer and stomach and pancreatic tumors.
1974
PRODUCT APPROVAL
Bristol Laboratories introduces cancer chemotherapy agent Blenoxane® (bleomycin sulfate) for the treatment of squamous cell cancers, head and neck cancers and Hodgkin’s and non-Hodgkin’s lymphomas.
1975
PRODUCT APPROVAL
Squibb researchers develop CAPOTEN® (captopril), the first ACE inhibitor for hypertension and later heart failure.
1976
PRODUCT APPROVAL
FDA approves CeeNU® (lomustine) and BICNU® (carmustine).
1978
CREATION OF CONVATEC
ConvaTec is formed by Squibb to market ostomy and wound therapy products.
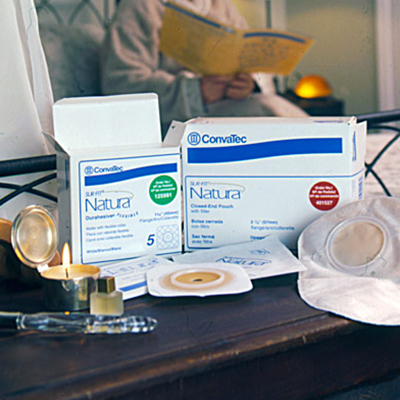

1978
CREATION OF CONVATEC
ConvaTec is formed by Squibb to market ostomy and wound therapy products.
1979
PRODUCT APPROVAL
PLATINOL® (cisplatin) becomes the first platinum-containing anti-cancer drug for testicular cancer.
1983
PRODUCT APPROVAL
The FDA approves Bristol Laboratories VePesid® (etoposide) for patients with testicular cancer that have not responded to standard chemotherapy.


1986
BRISTOL-MYERS RESEARCH COMPLEX OPENS
Bristol-Myers opens a state-of-the-art research complex in Wallingford, Connecticut, housing more than 800 scientists and support staff. In 1995, it is named the Richard L. Gelb Center for Pharmaceutical Research and Development after the former chairman and CEO.
1986
CELGENE FOUNDED
Celgene is founded by David Stirling, Ph.D. and Sol Barer, Ph.D. as an independent biotechnology company.


1986
CELGENE FOUNDED
Celgene is founded by David Stirling, Ph.D. and Sol Barer, Ph.D. as an independent biotechnology company.
1986
PRODUCT APPROVAL
BUSPAR, discovered by Bristol Myers neuroscience researchers, is approved for the treatment of generalized anxiety disorder.


1989
BRISTOL-MYERS AND SQUIBB MERGE
Bristol-Myers merges with Squibb, creating a global leader in the health care industry. The merger creates Bristol Myers Squibb, the world’s second-largest pharmaceutical enterprise. One of the first medicines the new company introduces is PARAPLATIN, approved for treating recurrent ovarian cancer.
1991
Product Approval
FDA approves TAXOL® (paclitaxel).
FDA approves VIDEX® (didanosine).
U.S. Full Prescribing Information including Boxed WARNINGS (PDF)
1993
THALIDOMIDE ADAPTATION
Scientists research and develop numerous compounds based on the structure of thalidomide, licensed by Celgene from Rockefeller University in 1992.


1993
THALIDOMIDE ADAPTATION
Scientists research and develop numerous compounds based on the structure of thalidomide, licensed by Celgene from Rockefeller University in 1992.
1994
PRODUCT APPROVAL
The FDA approves GLUCOPHAGE® (metformin hydrochloride).
U.S. Full Prescribing Information including Boxed WARNING (PDF)
1995
PRODUCT APPROVAL
ZERIT® (stavudine) is approved for treating HIV/AIDS.
1995
PRODUCT APPROVAL
FDA approves THALOMID® (thalidomide).
U.S. Full Prescribing Information including Boxed WARNINGS (PDF).


1997
RESEARCH CAMPUS OPENS
Bristol-Myers Squibb opens a 433-acre research campus in Hopewell, New Jersey.
1997
PRODUCT APPROVAL
The FDA approves two medicines co-developed with Sanofi: AVAPRO and PLAVIX® (clopidogrel bisulfate).
U.S. Full Prescribing Information for PLAVIX including boxed WARNING (PDF)
1998
NATIONAL MEDAL OF TECHNOLOGY
President Bill Clinton awards the National Medal of Technology — America’s highest honor for technological innovation — to Bristol-Myers Squibb "for extending and enhancing human life through innovative pharmaceutical research and development, and for redefining the science of clinical study through groundbreaking and hugely complex clinical trials that are recognized models in the industry."


1998
NATIONAL MEDAL OF TECHNOLOGY
President Bill Clinton awards the National Medal of Technology — America’s highest honor for technological innovation — to Bristol-Myers Squibb "for extending and enhancing human life through innovative pharmaceutical research and development, and for redefining the science of clinical study through groundbreaking and hugely complex clinical trials that are recognized models in the industry."


1999
SECURE THE FUTURE INITIATIVE ANNOUNCED
The Bristol Myers Squibb Foundation announces Secure the Future, a $100 million commitment to advance HIV/AIDS research and community outreach programs in seven African countries: Botswana, Namibia, Lesotho, Swaziland, Uganda, Burkina Faso and Tanzania.
2001
CHILDREN’S HOSPITAL OPENS
The Bristol-Myers Squibb Children’s Hospital opens as part of Robert Wood Johnson University Hospital in New Brunswick, New Jersey. The first freestanding children’s hospital in the state, it offers care without regard to a family’s ability to pay and offers more than 45 pediatric specialties.
DUPONT PHARMACEUTICALS PURCHASED
DuPont pharmaceuticals is acquired, strengthening Bristol Myers Squibb’s HIV and cardiovascular businesses while also adding medical imaging.


2001
CHILDREN’S HOSPITAL OPENS
The Bristol-Myers Squibb Children’s Hospital opens as part of Robert Wood Johnson University Hospital in New Brunswick, New Jersey. The first freestanding children’s hospital in the state, it offers care without regard to a family’s ability to pay and offers more than 45 pediatric specialties.
DUPONT PHARMACEUTICALS PURCHASED
DuPont pharmaceuticals is acquired, strengthening Bristol Myers Squibb’s HIV and cardiovascular businesses while also adding medical imaging.
2001
PRODUCT ACQUISITION
Bristol Myers Squibb gains SUSTIVA® (efavirenz) and COUMADIN® (warfarin sodium) from DuPont purchase.
U.S. Full Prescribing Information for COUMADIN including Boxed WARNING (PDF)
2001
PRODUCT APPROVAL
FDA approves FOCALIN® (dexmethylphenidate).
2002
PRODUCT APPROVAL
FDA approves ABILIFY® (aripiprazole).


2003
CELGENE AND ANTHROGENSIS MERGE
Celgene acquires Anthrogenesis and renames the unit Celgene Cellular Therapies (CCT).
2003
Children’s Clinical Center of Excellence
The first Children’s Clinical Center of Excellence—devoted to treating children with HIV/AIDS and their families—opens in Botswana. This joint effort with the Baylor International Pediatric AIDS Initiative is the first of four centers to become operational in Africa.


2003
Children’s Clinical Center of Excellence
The first Children’s Clinical Center of Excellence—devoted to treating children with HIV/AIDS and their families—opens in Botswana. This joint effort with the Baylor International Pediatric AIDS Initiative is the first of four centers to become operational in Africa.
2004
PRODUCT APPROVAL
FDA approves BARACLUDE® (entecavir).
U.S. Full Prescribing Information including Boxed WARNINGS (PDF)
FDA approves ERBITUX® (cetuximab).
2005
PRODUCT APPROVAL
FDA approves REVLIMID® (lenalidomide)—an IMiD® compound.
U.S. Full Prescribing Information including Boxed WARNINGS (PDF).


2007
CLINICAL CENTER FOR CHILDREN WITH HIV/AIDS OPENS
The clinical center at the Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson University Hospital in New Brunswick, New Jersey, is dedicated to the research and treatment of children’s immune system disorders and infectious diseases. The Bristol Myers Squibb Pediatric Infectious Disease and Immunology Center is made possible by a $5 million gift from the Bristol Myers Squibb Foundation.
2007
PRODUCT APPROVAL
FDA approves IXEMPRA® (ixabepilone).
2008
RESEARCH COLLABORATION
Celgene and Accerleron Pharma announce research collaboration to develop therapies for cancer and other rare diseases.

2008
RESEARCH COLLABORATION
Celgene and Accerleron Pharma announce research collaboration to develop therapies for cancer and other rare diseases.


2009
BRISTOL-MYERS SQUIBB ACQUIRES MEDAREX
Bristol Myers Squibb acquires Medarex, Inc., a biotech company and a partner since 2005. The acquisition significantly expands the company’s oncology and immunology pipeline.
2009
PRODUCT APPROVAL
FDA approves ONGLYZA® (saxagliptin).
2010
PRODUCT APPROVAL
FDA approves KOMBIGLYZE® XR (saxagliptin and metformin HCl extended‐release).
2010
PRODUCT ACQUISITION
Celgene gains ISTODAX® (romidepsin) from Gloucester Pharmaceuticals purchase.
2010
PRODUCT ACQUISITION
Celgene gains ABRAXANE® (paclitaxel protein-bound particles for injectable suspension) from Abraxis BioScience purchase.
U.S. Full Prescribing Information including Boxed WARNINGS (PDF).
2010
STRATEGIC COLLABORATION
Celgene and Agios Pharmaceutical, Inc. announce global strategic collaboration focused on targeting cancer metabolism.


2010
STRATEGIC COLLABORATION
Celgene and Agios Pharmaceutical, Inc. announce global strategic collaboration focused on targeting cancer metabolism.
2011
PRODUCT APPROVAL
FDA approves YERVOY® (ipilimumab).
U.S. Full Prescribing Information including Boxed WARNING regarding immune-mediated side effects (PDF)
FDA approves NULOJIX® (belatacept).
U.S. Full Prescribing Information including Boxed WARNING (PDF)
2012
PRODUCT APPROVAL


2013
INVESTING IN THE FUTURE
To expand its capabilities in development and manufacturing of biologics, the company announces a $250 million expansion of its manufacturing complex in Devens, Massachusetts.
2013
PRODUCT APPROVAL
FDA approves POMALYST® (pomalidomide)—an IMiD® compound.
U.S. Full Prescribing Information including Boxed WARNING (PDF)
2013
RESEARCH COLLABORATION
Celgene and bluebird bio enter into strategic research collaboration to develop novel therapies in oncology.


2013
RESEARCH COLLABORATION
Celgene and bluebird bio enter into strategic research collaboration to develop novel therapies in oncology.


2014
EXPANSION CONTINUES
Construction begins on a new, large-scale biologics manufacturing facility in Cruiserath, Ireland, to be built on the grounds of its existing bulk pharmaceutical manufacturing plant.
A new state-of-the-art office campus is planned for Lawrenceville, New Jersey, to replace leased facilities in Plainsboro and West Windsor.
2015
BRISTOL-MYERS SQUIBB NAMED A LEADING SUSTAINABLE COMPANY
Bristol-Myers Squibb is recognized as one of Dow Jones’ North America Index of Leading Sustainable Companies. Its efforts to promote economic, social and environmental sustainability are core to the mission and reflect its ongoing commitment to patients, employees and partners, the environment and communities around the world.
Bristol-Myers Squibb Foundation Celebrates 60th Anniversary
The Bristol-Myers Squibb Foundation marks a quarter of a century helping millions of people across the world overcome serious diseases.


2015
BRISTOL-MYERS SQUIBB NAMED A LEADING SUSTAINABLE COMPANY
Bristol-Myers Squibb is recognized as one of Dow Jones’ North America Index of Leading Sustainable Companies. Its efforts to promote economic, social and environmental sustainability are core to the mission and reflect its ongoing commitment to patients, employees and partners, the environment and communities around the world.
Bristol-Myers Squibb Foundation Celebrates 60th Anniversary
The Bristol-Myers Squibb Foundation marks a quarter of a century helping millions of people across the world overcome serious diseases.
2016
PRINCETON PIKE CAMPUS OPENS
This state-of-the-art campus in New Jersey features work space designed to foster greater innovation, productivity and collaboration.
PRIX GALIEN AWARD
Bristol-Myers Squibb is awarded the Prix Galien U.S. “Discovery of the Decade.” This is the third Prix Galien honor that the company has received, after being recognized for “Best Biotechnology Product” in 2012 and “Best Biotechnology Product” in 2015.


2016
PRINCETON PIKE CAMPUS OPENS
This state-of-the-art campus in New Jersey features work space designed to foster greater innovation, productivity and collaboration.
PRIX GALIEN AWARD
Bristol-Myers Squibb is awarded the Prix Galien U.S. “Discovery of the Decade.” This is the third Prix Galien honor that the company has received, after being recognized for “Best Biotechnology Product” in 2012 and “Best Biotechnology Product” in 2015.


2017
BRISTOL-MYERS SQUIBB CELEBRATES 130TH ANNIVERSARY
Chairman and CEO Giovanni Caforio rings the New York Stock Exchange’s Closing Bell in celebration of the company milestone.
2017
PRODUCT APPROVAL
2018
CELGENE ACQUIRES IMPACT BIOMEDICINES
Celgene gains INREBIC® (fedratinib) from IMPACT BioMedicines purchase.
U.S. Full Prescribing Information including Boxed WARNING (PDF)


2018
CELGENE ACQUIRES IMPACT BIOMEDICINES
Celgene gains INREBIC® (fedratinib) from IMPACT BioMedicines purchase.
U.S. Full Prescribing Information including Boxed WARNING (PDF)


2018
CELGENE ACQUIRES JUNO THERAPEUTICS
The acquisition contributes to Celgene’s stature as a premier cellular immunotherapy.
2019
PRODUCT APPROVAL
FDA approves REVLIMID® (lenalidomide) in combination with rituximab.
U.S. Full Prescribing Information including Boxed WARNING (PDF)
2019
COMBINATION REGIMEN APPROVAL
European Commission approves combination regimen for REVLIMID® (lenalidomide) and Imnovid® (pomalidomide).
U.S. Full Prescribing Information including Boxed WARNING (PDF)
2019
PRODUCT APPROVAL
2019
BRISTOL-MYERS SQUIBB ACQUIRES CELGENE
Bristol-Myers Squibb and Celgene come together to create a leading biopharma company positioned to address the needs of patients with serious diseases.

2019
BRISTOL-MYERS SQUIBB ACQUIRES CELGENE
Bristol-Myers Squibb and Celgene come together to create a leading biopharma company positioned to address the needs of patients with serious diseases.


2020
NEW CORPORATE BRAND AND VISION
As Bristol Myers Squibb builds for the future, we’re uniting around a single, powerful vision: transforming patients’ lives through science. Our new brand reflects the passion and precision we bring to pioneering life-saving medicines for people around the world and the compassion we bring to improving outcomes for the patients who need us most.
2020
PRODUCT APPROVALS
FDA approves ZEPOSIA® (ozanimod) and ONUREG® (azacitidine tablets).
ZEPOSIA® U.S Full Prescribing Information (PDF)
ONUREG® U.S. Full Prescribing Information (PDF)
2020
ACQUISITIONS
Bristol Myers Squibb acquires MyoKardia, strengthening the company’s leading cardiovascular franchise, and Forbius, adding a lead TGF-beta asset to the portfolio.

2020
ACQUISITIONS
Bristol Myers Squibb acquires MyoKardia, strengthening the company’s leading cardiovascular franchise, and Forbius, adding a lead TGF-beta asset to the portfolio.
2021
PRODUCT APPROVALS
FDA approves BREYANZI® (lisocabtagene maraleucel) and ABECMA® (idecabtagene vicleucel).
BREYANZI® U.S. Full Prescribing Information including Boxed WARNING (PDF)
ABECMA® U.S. Full Prescribing Information including Boxed WARNING (PDF)

2021
STRATEGIC COLLABORATION
Bristol Myers Squibb and Eisai announce global strategic collaboration for Eisai’s antibody drug conjugate.
2022
STRATEGIC COLLABORATION
Bristol Myers Squibb and Century Therapeutics enter a strategic collaboration to develop iPSC-derived allogeneic cell therapies.

2022
STRATEGIC COLLABORATION
Bristol Myers Squibb and Century Therapeutics enter a strategic collaboration to develop iPSC-derived allogeneic cell therapies.
2022
PRODUCT APPROVALS
FDA approves OPDUALAG™ (nivolumab and relatlimab-rmbw), CAMZYOS™ (mavacamten) and SOTYKTU™ (deucravacitinib).
OPDUALAG™ U.S. Full Prescribing Information (PDF)
CAMZYOS® U.S. Full Prescribing Information including Boxed WARNING (PDF)
SOTYKTU™ U.S. Full Prescribing Information (PDF)


2022
ACQUISITION
Bristol Myers Squibb acquires Turning Point Therapeutics, expanding our precision oncology portfolio.
2023
STRATEGIC COLLABORATION
Evotec and Bristol Myers Squibb extend and expand strategic neurodegeneration partnership.


2023
STRATEGIC COLLABORATION
Evotec and Bristol Myers Squibb extend and expand strategic neurodegeneration partnership.


2023
MANUFACTURING CAPABILITIES
Bristol Myers Squibb strengthens cell therapy capabilities by adding new U.S. manufacturing facility for viral vector production in Libertyville, Illinois.
2023
STRATEGIC COLLABORATION
Bristol Myers Squibb and Zenas BioPharma enter into a collaboration agreement to develop and commercialize obexelimab for autoimmune diseases in Japan, South Korea, Taiwan, Singapore, Hong Kong and Australia.
2023
ASSET ACQUISITION
BMS acquires global rights to Orum Therapeutics’ ORM-6151 program for all indications, including the treatment of patients with acute myeloid leukemia or high risk myelodysplastic syndromes.
2023
PRIX GALIEN AWARD
Bristol Myers Squibb is awarded the Prix Galien U.S. “Best Biotechnology Product.” This is the fourth Prix Galien honor that the company has received, after being recognized for “Discovery of the Decade” in 2016 and previously for “Best Biotechnology Product” in 2012 and 2015.
2023
PRODUCT APPROVAL


2024
ACQUISITION
Bristol Myers Squibb strengthens and diversifies oncology portfolio with acquisition of Mirati Therapeutics.
2024
STRATEGIC COLLABORATION
Bristol Myers Squibb and SystImmune announce a global strategic collaboration agreement for the development and commercialization of BL-B01D1.
2024
ACQUISITION
Bristol Myers Squibb adds premier radiopharmaceutical platform with acquisition of RayzeBio.


2024
ACQUISITION
Bristol Myers Squibb adds premier radiopharmaceutical platform with acquisition of RayzeBio.


2024
ACQUISITION
Bristol Myers Squibb strengthens neuroscience portfolio with acquisition of Karuna Therapeutics
