Path to a protein degrader
Targeted protein degradation at Bristol Myers Squibb
Bristol Myers Squibb’s investigation of protein degradation began more than two decades ago. Building on this legacy and scientific expertise, Bristol Myers Squibb is discovering and developing novel degraders in blood cancers, solid tumors, immune-mediated disease and other therapeutic areas with high unmet need.
Protein degradation is the process by which proteins are naturally destroyed in a cell. With targeted protein degradation, researchers are harnessing the cell's own degradation machinery, for example ubiquitin ligase (E3) enzymes, to degrade therapeutically relevant proteins that were previously considered "undruggable".
Learn more about the basics of protein degradation here.
The science behind the discovery and development of protein degraders has evolved over time.
Early protein degraders were discovered and developed based on the observed effect within patients and without a clear picture of how they worked in the body.
Today, newer protein degraders are being designed and tailor-made to specifically target disease causing proteins.
Researchers are also applying cutting edge tools and technologies, such as artificial intelligence and machine learning, to analyze vast data and chemical libraries and accelerate drug discovery and development timelines.
Discovery and development
Researchers at Bristol Myers Squibb are leveraging three different modalities of targeted protein degradation – molecular glues, also called CELMoDTM agents, ligand-directed degraders (LDDs) and degrader antibody conjugates (DACs). This three-pronged approach provides more opportunities for scientific breakthroughs across a broad range of diseases.
Each of these protein degrader modalities possess unique attributes and are discovered or created with their own process, sophisticated technology and research expertise.
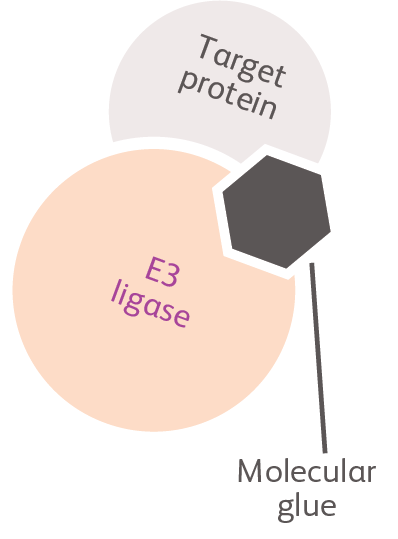
Molecular glues (CELMoD Agents)
CELMoD agents are molecules that act as molecular glue to alter the protein-binding properties of cereblon (an important component of the cellular protein degradation machinery) to promote interaction with and degradation of target proteins.
It all begins with the CELMoD library
- Bristol Myers Squibb has built an industry-leading CELMoD library using computational and medicinal chemistry
- CELMoD agents from the library are screened in relevant cells in the lab
- The disappearance of proteins is measured
Choosing a potential target
- Researchers examine the proteins that disappeared to determine their relevance in various diseases
- This identifies which CELMoD agent/target pairs researchers will take forward to the optimization step
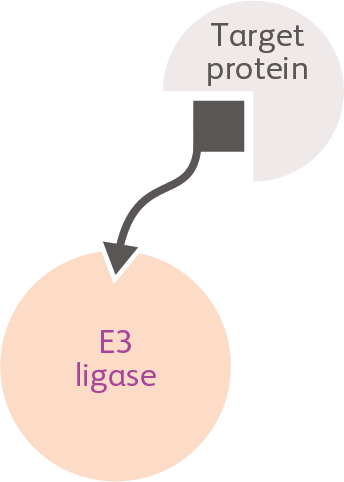
Ligand-directed degraders (LDDs)
LDDs are 3-component molecules (a target binder and a ligase binder connected by a linker) engineered to bring together target proteins with key components of the cellular protein degradation machinery, redirecting the machinery to degrade the target proteins.
It all begins with a target
- Researchers identify target proteins implicated in a disease with the aim of removing them from the cells
Building an LDD
- Scientists begin building the LDD starting with the end that interacts with the identified target of interest
- This molecule is often found using internal screening platforms
- The linker, or middle component, is then attached
- Finally, a molecule on the opposite end that binds the protein degradation cellular machinery is connected to the linker
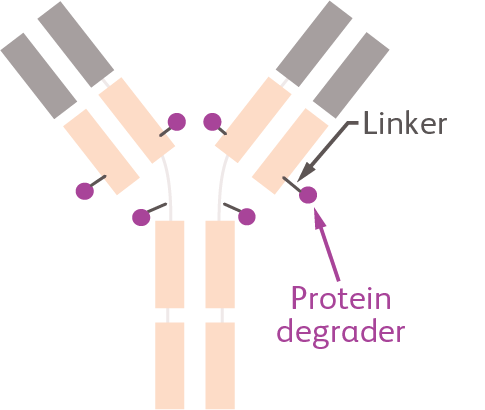
Degrader antibody conjugates (DACs)
DACs consist of 3 elements: a monoclonal antibody that specifically targets cancer cells, the payload (such as a CELMoD protein degrader), and a linker that connects the two.
It all begins with the right 'partnership'
DACs combine a clinically-validated tumor-targeting antibody with a protein degrader molecule with the goal to enhance efficacy and tolerability compared to a protein degrader on its own.

Optimization
All potential protein degraders are tested and optimized through various preclinical studies and processes.

Clinical Trials
If the above process yields a possible candidate that has the potential to benefit patients, that candidate is then tested in clinical trials using human volunteers.
Clinical trials help researchers to understand whether the potential therapeutic is safe and effective.
Bristol Myers Squibb is building on decades of unique research and clinical experience to advance the field of targeted protein degradation and transform patient outcomes in diseases with serious unmet need.