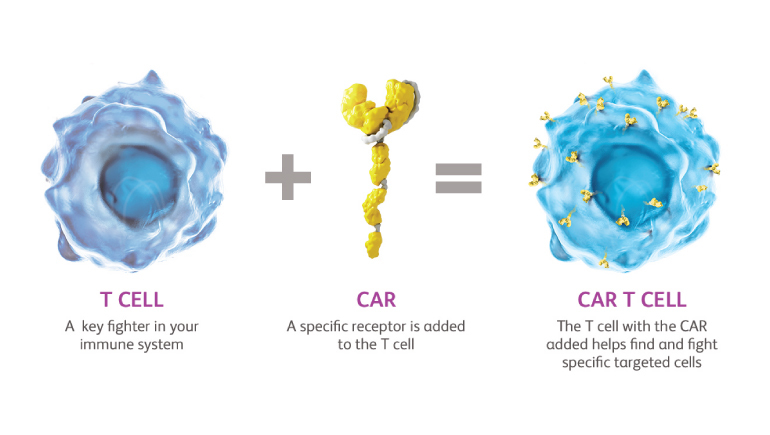
The 244,000 square-foot Devens facility integrates the latest cell therapy manufacturing equipment and most recent and impactful digital technology, including Automated Media Production, which reduces the time to prepare complex media formulations.
The facility will add an additional several hundred jobs to the Devens campus, with nearly 150 new employees already on board at the facility. This latest milestone opens the doors to local employees as they prepare the facility to begin manufacturing next year.
The cell therapy manufacturing facility represents the second significant expansion of the 89-acre Devens site. The site began operations in 2009 and focused on large-scale, bulk biologics manufacturing in its early years. A 200,000 square-foot expansion completed in 2016 added biologics process development and clinical manufacturing capabilities.
“The new facility at the existing Devens site is a critical component of expanding our cell therapy manufacturing footprint and capabilities,” said senior director and Devens Cell Therapy Facility Program Lead Mike Hausladen. “This will allow us to prepare for the approval and launch of new cell therapies to help more patients across the globe.”
The Devens site is part of Bristol Myers Squibb’s growing presence in Massachusetts, which is home to a robust life sciences ecosystem. The company also operates two R&D facilities in Cambridge, MA, and will be bringing those two sites together into a new building at Cambridge Crossing in 2023.
Leiden, Netherlands